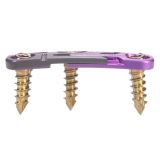
Ürün Açıklaması
Ant-Cer II bölümlenmiş bir tasarım ve greft üzerinde sabit eksenel yük sunmak için kontrollü, tek yönlü dişli çark özellikleri dinamik servikal plakadır. Sonuç olarak, bu cihaz başarılı kemik yeniden teşvik etmek için Wolff kanunu geçerlidir. Tek düzeyli ve çok düzeyli bir tabak tasarımları mevcuttur, Ant-Cer II patentli tek parça, sıfır adım bulunmaktadır SecureRing ®kilitleme teknolojisi.
Özellikler
- Kontrollü cırcırlı Teknoloji greft üzerinde sabit eksenel yük sunar
- 2mm eksenel seviye başına yerleşmek için izin verir
- Segment tasarımı göç önler
- Önceden yüklenmiş sınırlayıcı plakası daraltma veya implantasyon öncesinde disassociating plakası önler
- Çeviri plaka aracılığıyla gerçekleşmesi için Kısıtlı vida deliklerini izin vermemesi ve vidaları geçiş yaparak
- SecureRing tek parça, sıfır-adım kilitleme mekanizması ile teknoloji
- Için kullanılan aynı çekirdek araç seti SC-AcuFix ® plakaları
ZS-SA0700-02_A
Device Description
The Zimmer Spine SC-AcuFix Anterior Cervical Plate System components are temporary implants and associated instruments that are used to stabilize the cervical spine during the development of a solid spinal fusion in patients with degenerative disease, trauma (including fractures), and tumor pathology. The SC-AcuFix System consists of single and multi-segmented titanium bone plates of various sizes and lengths, titanium bone screws in various diameters and lengths, and instrumentation for plate insertion. Fixation is provided by the insertion of bone screws through the two openings at each end of a plate segment into the vertebral bodies of the cervical spine. Fixation of the screws to the plate is accomplished by seating into the SecureRing® screw retention mechanism. Screws may also be inserted into additional adjacent screw holes of multi-segment plates if needed.
Indications
The SC-AcuFix Ant-Cer II Dynamic Anterior Cervical Plate System is indicated for use in the temporary stabilization of the cervical spine (C2-C7) during the development of solid spinal fusion in patients with instability caused by the following:
- Degenerative disc disease (DDD) – as defined by neck pain of discogenic origin with degeneration of the disc confirmed by patient history and radiographic studies;
- Trauma (including fractures);
- Tumor;
- Spondylolisthesis;
- Spinal stenosis;
- Deformity (i.e., scoliosis, kyphosis, lordosis);
- Pseudarthrosis; and
- Failed previous fusions.
Contraindications
- Presence of overt infection and/or localized inflammation.
- Rapid joint disease, bone absorption, osteopenia, and/or osteoporosis.
- Suspected or documented metal allergy or intolerance.
- Any patient having inadequate tissue coverage over the operative site.
- Any time implant utilization would interfere with anatomical structures or expedited physiological performance, such as impinging on vital structures.
- Severe comminuted fractures such that segments may not be maintained in satisfactory proximate reduction.
- Use in displaced, non-reduced fractures with bone loss.
- The presence of marked bone absorption or severe metabolic bone disease that could compromise the fixation achieved.
- Any other medical or surgical condition which would preclude the potential benefit of surgery, such as elevation of sedimentation rate unexplained by other diseases, elevation of white blood count (WBC), fever, leukocytosis or a marked left shift in the WBC differential count.
- The physical contact of the SC-AcuFix Ant-Cer II Dynamic Anterior Cervical System implants with metal implant made of anything other than implant grade titanium, such as stainless steel (ASTM F138) or MP35 N, or other dissimilar metal.
- Situations with the absence or compromise of significant stabilizing elements.
- Use in the presence of any neural or vascular deficits or other compromising pathology, which may be further injured by device intervention.
Warnings
Following are specific warnings, precautions, and adverse effects, which should be understood by the surgeon and explained to the patients. These warnings do not include all adverse effects, which can occur with surgery in general, but are important considerations particular to metallic internal fixation devices. General surgical risks should be explained to the patient prior to surgery.
- In the U.S.A., this product has labeling limitations.
- This device is not approved for screw attachment or fixation to the posterior elements(pedicles) of the cervical, thoracic, or lumbar spine.
- Potential risks identified with the use of this device system, which may require additional surgery, include:
Precautions
- CORRECT HANDLING OF THE IMPLANT IS EXTREMELY IMPORTANT. Contouring of the metal implants should only be done with proper equipment. It is recommended that contouring be gradual and that great care be used to avoid any notching, scratching or reverse bending of the devices when contouring. Alterations will produce defects in surface finish and internal stresses which may become the focal point for eventual breakage of the implant.
- REMOVAL OF THE IMPLANT AFTER HEALING. Metallic implants can loosen, fracture, corrode, migrate, possibly increase the risk of infection, cause pain, or stress shield bone even after healing, particularly in young, active patients. The surgeon should carefully weigh the risk versus benefits when deciding whether to remove the implant. Implant removal should be followed by adequate postoperative management to avoid refracture. If the patient is older and has a low activity level, the surgeon may choose not to remove the implant thus eliminating the risk involved with a second surgery.
- ADEQUATELY INSTRUCT THE PATIENT. Postoperative care and the patient’s ability and willingness to follow instructions are one of the most important aspects of successful bone healing. The patient must be made aware of the limitations of the implant and follow the post-operative care regimen as instructed by his or her physician.
- DO NOT ALTER OR MODIFY ANY SC-AcuFix SYSTEM INSTRUMENT. Repairs should only be accomplished by the manufacturer. The SC-AcuFix System is only a temporary implant used for the correction and stabilization of the cervical spine. A successful result is not achieved in every surgical case. Bone grafting must be part of the spinal fusion procedure in which the SC-AcuFix System is used.
- All implants and some instruments are intended for single use only; refer to the product label to determine if the instrument is single use only. Single use devices should not be re-used. Possible risks associated with re-use of single-use devices include:
- Mechanical malfunction
- Transmission of infectious agents
- Device corrosion with localized tissue reaction and pain.
- Device migration which may result in injury to soft tissue, visceral organs or joints.
- Loosening or disassembly of implant resulting in additional injury.
- Bending, loosening or breaking of the implant making removal difficult, impractical.or impossible.
- Abnormal sensations, discomfort or pain.
- Increased risk of infection.
- Bone loss due to stress shielding.
Possible Adverse Effects
Occurrence of any adverse effects may require re-operation and removal of the implant. Adverse effects may include but not be limited to:
- Early or late loosening of the components.
- Disassembly, fretting, loosening, bending, breakage and/or migration of any component or component portion.
- Foreign body reaction to the implants.
- Pressure on the skin from component parts where there is inadequate tissue coverage over the implant, causing skin irritation.
- Early or late infection.
- Vertebral body fracture at, above, or below the level of surgery.
- Implants cutting through bone, especially soft osteoporotic, osteopenic, or cancellous bone.
- Bone forming around the implant, making removal difficult or impossible.
- Non-union (pseudarthrosis) or bone fracture.
- Post-operative change in spinal curvature, loss of correction, height, and/or reduction.
- Neurovascular compromise including radiculopathy, paralysis, or other types of serious injury causing pain and disability.
- Hemorrhage of blood vessels.
- Cessation of growth of the operated portion of the bone.