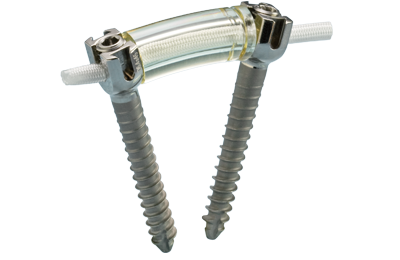
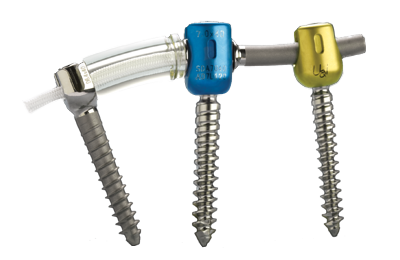
Ürün Açıklaması
Dynesys Dinamik Stabilizasyon Sistemi omurga stabilizasyonu daha fizyolojik bir sistem olarak tasarlanmıştır. Sistem:
- Katı olmayan bir malzeme kullanılarak daha doğal bir anatomik mevki içinde segmentleri tutar
- Mümkün olduğunca doğal anatomik yapıları korur
- (“Iç atel”) spinal hareket Kısıtların
Zimmer Omurga üç dinamik dengeleme seçenekleri sunar:
Dynesys LIS Dinamik Stabilizasyon Sistemi
- Orijinal Dynesys küresel klinik deneyim sağlam bir geçmişi olan Sistem
- Tüm boyutları mevcuttur Standart ve HA kaplamalı vidalar
Dynesys Top-Loading Spinal Sistem
- Bir üst yükleme, kanüle vida seçeneği sunar
- Tüm boyutları mevcuttur Standart ve HA kaplamalı vidalar
- Daha az invaziv paraspinal yaklaşım sağlar
- Kord ve boşluk doğrudan implantasyonu için benzersiz enstrümantasyon kullanır
Zimmer ® DTO ® İmplant ve OPTIMA ™ * ZS Geçiş Vida
- Kombine kord-çubuk yapı dinamik stabilizasyon sistemine sert bir geçiş olanağı sunar
- Ile uyumlu Dynesys LIS ve Dynesys Top-Loading sistemleri.
Bu cihazın güvenliğini ve etkinliğini füzyon olmaksızın spinal stabilizasyon kullanım amacı için kurulmamıştır. Bu cihaz, sadece kemik grefti ile füzyon her Enstrümante düzeyde yürütülür edilirken kullanılmak üzere tasarlanmıştır.
Dinamik Stabilizasyon Sistemleri Faydaları
- Hasta Özgü
– Ameliyatta her hastanın doğal anatomik yapısını yerleştirmek için boşluk ölçmek ve kesmek - Daha Yük-To Be tasarlanan Paylaşımı vs Taşıyıcı
stres koruyucu en aza indirmek için tasarlanmıştır –
– fleksiyon ve ekstansiyon hareket kontrollü bir aralığı sağlar - Anatomi Koruma
– Far-lateral, düşük profilli vida yerleştirme faset eklemleri korur
– Wiltse paraspinal Cerrahi yaklaşım posterior anatomisine kesintileri azaltır - Çok yönlülük
– Zimmer DTO implant cerrahlar bitişik seviyelerde dejenerasyon farklı aşamalarında tedavi sağlar
* OPTIMA ™ ZS Spinal Fiksasyon Sistemi U & i Corporation’ın bir ticari markasıdır.
ZS-SA0700-07_A
Indications
Dynesys LIS System, Dynesys Top-Loading System & Zimmer DTO System
When used as a pedicle screw fixation system in skeletally mature patients, the Dynesys Spinal System is intended to provide immobilization and stabilization of spinal segments as an adjunct to fusion in the treatment of the following acute and chronic instabilities or deformities of the thoracic, lumbar, and sacral spine: degenerative spondylolisthesis with objective evidence of neurologic impairment, and failed previous fusion (pseudoarthrosis).In addition, when used as a pedicle screw fixation system, the Dynesys Spinal System is indicated for use in patients:
- Who are receiving fusions with autogenous graft only;
- Who are having the device fixed or attached to the lumbar or sacral spine;
- Who are having the device removed after the development of a solid fusion mass.
Contraindications
Dynesys LIS System, Dynesys Top-Loading System & Zimmer DTO System
Contraindications of the Dynesys Spinal System are similar to other commercially available posterior spinal fixation systems. Contraindications include but are not limited to the following:
- Use in the cervical spine;
- Active systemic or local infection;
- Obesity;
- Pregnancy;
- Mental illness;
- Severe osteoporosis or osteopenia;
- Sensitivities/allergy to metals; polymers, polyethylene, polycarbonate urethane and polyethylene terephthalate;
- Alcohol or drug abuse;
- Patient unwilling or unable to follow postoperative instructions;
- Soft tissue deficit not allowing sound closure;
- Any medical or physical condition that would preclude the potential benefit of spinal implant surgery;
- Congenital abnormalities, tumors or other conditions that would prevent secure component fixation that has the potential to decrease the useful life of the device;
- Any medical or mental condition which would exclude the patient at high risk from surgery of this severity;
- For pedicle screw cases, inadequate pedicles of the thoracic, lumbar, and sacral vertebrae.
Warnings
Dynesys LIS System, Dynesys Top-Loading System & Zimmer DTO System
The safety and effectiveness of the Dynesys Spinal System and the Zimmer DTO Implant have not been established for spinal indications beyond those stated in the Indications section.
The safety and effectiveness of this device has not been established for the intended use of spinal stabilization without fusion. This device is only intended to be used when fusion with bone graft is being performed at all instrumented levels.
For a complete list of Warnings and Precautions for the OPTIMA ZS Spinal System, including the OPTIMA ZS Transition Screw, refer to the Instructions for Use for that system.
Precautions
Dynesys LIS System, Dynesys Top-Loading System & Zimmer DTO System
Only experienced spinal surgeons with specific training in the use of the Dynesys Spinal System, the Zimmer DTO Implant and the OPTIMA ZS Spinal system should perform the implantation of these systems. This is due to the technically demanding procedure presenting a risk of serious injury to the patient. These systems should only be used with instrumentation specifically designed for each system. Refer to the respective surgical techniques to determine which instruments should be used for each step of the surgical procedure.
Unless the Zimmer DTO Implant is being used, components of spinal fixation systems other than Zimmer Companies should not be used with the components of the Dynesys Spinal System. Only the OPTIMA ZS Spinal System, including the OPTIMAZS Transition Screw, may be used in combination with the Zimmer DTO Implant.
No component of the Dynesys Spinal System and the Zimmer DTO Implant should be reused or re-sterilized.
The Dynesys Spinal System and the Zimmer DTO Implant are intended to be used with bone graft, which is required to provide additional spinal support. A successful result is not always achieved in every surgical case.
The patients should be made aware that a successful result, as defined by reduced pain, increased function and the establishment of solid fusion, is not always achieved in every surgical case. Proper patient selection will greatly affect the results. Patients who smoke have been shown to have an increased incidence of non-union. These patients should be informed of this increased risk and counselled to discontinue tobacco use prior to and immediately after surgery. Obese, malnourished, and/or alcohol abuse patients are also poor candidates for spinal fusion. Patients with poor muscle tone and bone quality, and/or nerve paralysis are also poor candidates for spinal fusion. The use of autogenous bone graft has been shown to provide superior results compared to the use of allograft bone graft material.
In addition to the above specified warnings and precautions, general surgical risks should be explained to the patient prior to surgery.
Complications and Possible Adverse Events
Dynesys LIS System, Dynesys Top-Loading System & Zimmer DTO System
Prior to surgery, patients should be made aware of the following possible adverse effects in addition to the potential for additional surgery to correct these effects:
- Loosening, disassembly, bending or breakage of components;
- Tissue sensitivity to implant material;
- Potential for skin breakdown and/or wound complications;
- Non-union or delayed union;
- Infection;
- Nerve damage, including loss of neurologic function, dural tears, paralysis, paresthesia, and cerebral spinal fluid leakage;
- Fracture of vertebrae;
- Foreign body reaction (allergic) to components or debris;
- Loss of fixation;
- Vascular or visceral injury;
- Change of normal spinal curvature;
- Gastrointestinal, urological and/or reproductive system compromise;
- Pain or discomfort;
- Bursitis;
- Decrease in bone density due to stress shielding;
- Loss of bone or fracture of bone above or below the level of surgery;
- High removal torques may be encountered with the use of the hydroxyapatite coated screw;
- Bone graft donor site pain, fracture, and/or delayed wound healing;
- Restriction of activities;
- Lack of effective treatment of symptoms for which surgery was intended;
- Death.