Product Description
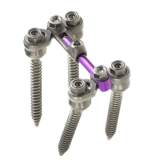
Zimmer Spine’s ST360° Spinal Fixation System is an offset solution that combines the flexibility of polyaxial screws with the versatility of lateral connectors. Designed for easy assembly and backed by a long clinical history, this proven system allows surgeons to adapt to challenging patient anatomies with minimal rod contouring. As a result, the ST360° System minimizes excessive intraoperative loading to the spine. Experience more surgical flexibility with the ST360° System — another winning solution from Zimmer Spine.
Features
- The ST360° System requires less rod contouring and enables assembly of the rod and lateral connectors outside the surgical site
- Top-loading rods provide an in-line option for one-level cases that combines the flexibility of polyaxial screws with the benefits of a low-profile, low-metal-volume construct
- The ST360° System adapts to patient anatomy by decreasing stress and loading during construct assembly
- Offset connectors circumvent healthy facet joints in adjacent levels, minimizing impingement
- Lateral connectors accommodate variations in anatomy and accept both 5.5mm and 6.35mm rods, maximizing intraoperative flexibility
- Guide pins facilitate placement of assembled construct
ZS-SA0700-19_A
Device Description
The ST360°® Spinal Fixation System is a temporary implant system used to correct spinal deformity and facilitate the biological process of spinal fusion. This system is intended for posterior use in the thoracic, lumbar and sacral areas of the spine. Implants of this system consist of hooks and/or screws connected to rods and are intended to be removed after solid fusion has occurred. This system includes polyaxial screws of varying diameters and lengths, fixed screws of varying diameters and lengths, rods in varying lengths, hooks in varying designs, fixed and adjustable transverse connectors. The implants in this system are manufactured from titanium alloy (Ti-6Al-4V), conforming to ASTM F-136.
The ST360° Spinal Fixation System is a spinal rod, screw and hook system. This system’s polyaxial and fixed screws, hooks, and connectors can be rigidly locked into a wide range of configurations, therefore, allowing each construct to be formed to the needs of an individual patient. Rods of this system are shaped intraoperatively to correct or maintain proper spinal curvature.
Torque limiting instruments are designed to deliver a preset torque value when rotated in the clockwise direction. When the preset torque value is reached the instrument is designed to release. The handle will rotate approximately 30 degrees and the instrument is then reset to deliver the preset torque value again.
Indications
The ST360°® Spinal System is intended for posterior, noncervical pedicle and nonpedicle fixation for the following indications: degenerative disc disease (DDD) (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies); spondylolisthesis; trauma (i.e., fracture or dislocation); spinal stenosis; deformities or curvatures (i.e., scoliosis, kyphosis, and/or lordosis), tumor; pseudoarthrosis, and failed previous fusion. Pedicle screw fixation is limited to skeletally mature patients. When used as a hook and sacral screw system, the ST360° Spinal Fixation System is intended for use in the treatment of degenerative disc disease (as defined by chronic back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), idiopathic scoliosis, spondylolisthesis, kyphotic or lordotic deformity of the spine, loss of stability due to tumors, spinal stenosis, vertebral fracture or dislocation, pseudoarthrosis, and previous failed spinal fusion. When used for this indication, screws of theST360° Spinal Fixation System are intended for sacral iliac attachment only. Hook and transverse connectors of the system are intended for posterior thoracic and/or lumbar use only. As a whole, the levels of use for hook and sacral screw fixation of this system are T1 to the sacrum.
Contraindications
Contraindications of the ST360° Spinal Fixation System are similar to other commercially available posterior spinal fixation systems of similar design and material. Contraindications include but are not limited to the following:
- Use in the cervical spine
- Active systemic or local infection
- Obesity
- Pregnancy
- Mental illness
- Severe osteoporosis or osteopenia
- Metal sensitivity/allergies to the implant material
- Alcohol or drug abuse
- Patients are unwilling or unable to follow postoperative instructions
- Soft tissue deficit not allowing wound closure
- Any medical or physical condition that would preclude the potential benefit of spinal implant surgery
- Congenital abnormalities, tumors or other conditions that would prevent secure component fixation that has the potential to decrease the useful life of the device
- Any medical or mental condition which would exclude the patient or put the patient at high risk from surgery of this severity
- For pedicle screw cases, inadequate pedicles of the thoracic, lumbar, and sacral vertebrae
Warnings
The safety and effectiveness of pedicle screw spinal systems have been established only for spinal conditions with significant mechanical instability or deformity requiring fusion with instrumentation. These conditions are significant mechanical instability or deformity of the thoracic, lumbar, and sacral spine secondary due to degenerative disc disease (DDD) (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis; trauma (i.e., fracture or dislocation), spinal stenosis, deformities or curvatures, (i.e., scoliosis, kyphosis, and/or lordosis), tumor, pseudoarthrosis, and failed previous fusion. Pedicle screw fixation is limited to skeletally mature patients. The safety and effectiveness of these devices for any other conditions are unknown.
Potential risks identified with the use of this system that may require additional surgery include device component fracture, loss of fixation, non-union, fracture of the vertebrae, neurologic injury, and vascular or visceral injury.
The ST360° Top Loading rods are intended for single-level use only.
Precautions
The implantation of pedicle screw spinal systems should be performed only by experienced spinal surgeons with specific training in the use of this pedicle screw spinal system because this is a technically demanding procedure presenting a risk of serious injury to the patient. This system should only be used with instrumentation specifically designed for this system.
The ST360° Spinal Fixation System can only be used in conjunction with the components from the Silhouette Spinal System®. Components of competitive spinal fixation systems should not be used with components of the ST360° Spinal Fixation System due to the potential for accelerating the corrosion process by the mixing of dissimilar materials.
No component of the ST360° Spinal Fixation System should be reused after being removed from the body. An implant should never be re-sterilized after contact with body tissue or body fluids. All implants and some instruments are intended for single use only; refer to the product label to determine if the instrument is intended for single use only. Single use devices should not be re-used. Possible risks associated with re-use of single use devices include:
- Mechanical malfunction
- Transmission of infectious agents
Complications and Possible Adverse Effects
Prior to surgery, patients should be made aware of the following possible adverse effects in addition to the potential for additional surgery to correct these effects:
- Loosening, disassembly, bending or breakage of components
- Tissue sensitivity to implant material
- Potential for skin breakdown and/or wound complications
- Non-union or delayed union
- Infection
- Nerve damage, including loss of neurologic function, dural tears, paralysis, paresthesia, and cerebral spinal fluid leakage
- Fracture of vertebrae
- Loss of fixation
- Vascular or visceral injury
- Change of normal spinal curvature
- Gastrointestinal, urological and/or reproductive system compromise
- Pain or discomfort
- Bursitis
- Decrease in bone density due to stress shielding
- Loss of bone or fracture of bone above or below the level of surgery
- Bone graft donor site pain, fracture, and/or delayed wound healing
- Restriction of activities
- Lack of effective treatment of symptoms for which the surgery was intended
- Death