Ürün Açıklaması
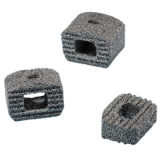
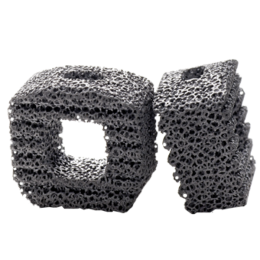
Bu servikal füzyon cihaz porozite ve dayanım arasındaki mükemmel bir denge sağlar. TM-S ile yapılan sadece servikal spinal implant Trabeküler Metal Malzeme. Bu, cihazın yer değiştirmesini ve çıkarma, hem de potansiyel stres-koruyucu minimize yük paylaşımı, artırabilir bir elastisite düşük modüllü önlemeye yardımcı olmak için, yüksek bir sürtünme katsayısına sahiptir. % 80 ile tutarlı bir açık gözenek yapısı arasında, ortalama gözenek ile, malzeme aynı zamanda ve vaskülarizasyon-büyüme boney için mükemmel bir ortam sağlar spongiöz kemik yapısı ve mekanik özellikleri, benzer şekilde tasarlanmıştır.
Özellikler
Kansellöz kemik fiziksel ve mekanik özellikleri benzer şekilde tasarlanmış, tutarlı, açık gözenek yapısı ile% 80’e varan ortalama gözeneklilik
Potansiyel stres koruyucu minimize yük paylaşımı, artırabilir elastikiyet Düşük modüllü
Cihaz migrasyonu ve sınırdışı önlemek için sürtünme katsayısı yüksek
ZS-SA0700-23_A
Device Description
Indications
Contraindications
- Active local infection in or near the operative region.
- Active systemic infection and/or disease.
- Severe osteoporosis or insufficient bone density, which in the medical opinion of the physician precludes surgery or contraindicates instrumentation.
- Spinal conditions other than cervical DDD.
- Prior surgical procedure using the desired operative approach.
- Current metastatic tumors of the vertebrae adjacent to the implant.
- Known or suspected metal sensitivity.
- Endocrine or metabolic disorders known to affect osteogenesis (e.g., Paget's disease, renal osteodystrophy, hypothyroidism).
- Systemic disease that requires the chronic administration of nonsteroidal anti-inflammatory or steroidal drugs.
- Significant mental disorder or condition that could compromise the patient's ability to remember and comply with preoperative and postoperative instructions (e.g., current treatment for a psychiatric/psychosocial disorder, senile dementia, Alzheimer's disease, traumatic head injury).
- Neuromuscular disorder that would engender unacceptable risk of instability, implant fixation failure, or complications in postoperative care. Neuromuscular disorders include spina bifida, cerebral palsy, and multiple sclerosis.
- Pregnancy.
- Patients unwilling to follow postoperative instructions, especially those in athletic and occupational activities.
- Morbid obesity.
- Symptomatic cardiac disease.
- Skeletal immaturity.
- Grossly distorted anatomy.
- Conditions other than those indicated.
Warnings
- Surgery is not always successful. Preoperative symptoms may not be relieved or may worsen. Surgical knowledge of the procedure and the device are important, as is patient selection. Patient compliance is also important. Tobacco and alcohol abuse may lead to unsuccessful results.
- Reuse of a single use device that has come in contact with blood, bone, tissue or other body fluids may lead to patient or user injury. Possible risks associated with reuse of a single use device include, but are not limited to, mechanical failure and transmission of infectious agents.
- Appropriate device selection is crucial to obtain proper fit and to decrease the stress placed on the implant.
- Components of competitive spinal systems should not be used with the TM-S Fusion Device.
- Delayed healing can lead to fracture or breakage of the implants due to increased stress and material fatigue. Patients must be fully informed of all the risks associated with the implant and the importance of following postoperative instructions regarding weight bearing and activity levels to facilitate proper bone growth and healing.
- The implant must be handled carefully following the manufacturer’s instructions to prevent damage to the implant.
- Implants must not be modified or otherwise processed in any way.
- Care must be taken to avoid using dissimilar metals in contact with one another as corrosion may occur. Additional fixation instrumentation that is used to stabilize the affected level must be made of compatible materials, such as titanium or titanium alloy. Corrosion may accelerate metal fatigue and lead to failure of the implant.
- Once a device has been implanted, it must never be reused. If the package is damaged or opened but the device is not used, or if the expiration date has passed, the device must be returned to Zimmer. The device must not be resterilized by the end user.
- The surgeon must be familiar with the appropriate technique to implant the supplemental internal fixation and the appropriate hardware.
- MRI Compatibility11.1. The patient must be told that implants can affect the results of computer tomography (CT) or magnetic resonance imaging (MRI) scans. 11.2. The TM-S Fusion Device has not been evaluated for safety or compatibility in the MR environment. 11.3. The TM-S Fusion Device has not been tested for heating or migration in the MR environment.
- This surgical procedure requires the use of supplemental fixation systems to stabilize the fusion site.
Precautions
- The implantation of an intervertebral body fusion device should be performed only by experienced spinal surgeons with specific training in the use of this device because this is a technically demanding procedure presenting a risk of serious injury to the patient.
- The surgeon must have a thorough knowledge of the mechanical and material limitations of surgical implants made of Trabecular Metal and be thoroughly familiar with the surgical technique for implanting the TM-S Fusion Device for the given Indications for Use.
- Based on the fatigue testing results, the physician/surgeon should consider the level of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact on the performance of the system.
- The surgeon should be familiar with the various devices and instruments and verify that all are available before beginning the surgery. Additionally, the packaging and implant should be inspected for damage prior to implantation.
- In the event that removal of the implant is considered (e.g. due to loosening, fracture, migration of the implant; infection; increased pain, etc.), the risks versus benefits must be carefully weighed. Such events can occur even after healing, especially in more active patients. Appropriate postoperative care must be given following implant removal to avoid further complication.
- The surgeon must be thoroughly familiar with the options for supplemental internal fixation systems and the associated surgical techniques.
- Implants must be fully seated within the inserter prior to use. Care must be taken not to over-tighten the implant-inserter assembly. Additionally, care must be taken not to manipulate the inserter implant interface in a way not recommended by the surgical technique.
- The surgeon must ensure that the implant is properly seated prior to closing of the soft tissue.
- Extreme caution must be used around the spinal cord, nerve roots and blood vessels.
- Postoperative care instructions are extremely important and must be followed carefully. Non-compliance with postoperative care instructions could lead to failure of the device, and the possibility of additional surgery to remove the device.
- The patient should limit activities that result in overhead lifting, repetitive neck bending (especially neck extension) and heavy lifting until a physician determines solid bony fusion is achieved.
- An orthotic brace may be worn following surgery for support. The attending physician, based upon each patient’s clinical progress, will determine whether a brace is appropriate and, if necessary, the length of time the brace is prescribed.
- Non-steroidal anti-inflammatory and steroidal drugs should be avoided for at least 45 days, or as directed by a physician, postoperatively.
Possible Adverse Effects
- Potential complications associated with the device itself include:
- Bone lysis associated with particulate metal debris,
- Device bending,
- Device fracture,
- Device loosening,
- Device migration.
- Failure to properly fill and compress the graft material into the implant may result in delayed healing and/or nonunion.
- If either the iliac crest, fibula or rib are utilized as a secondary donor site for bone graft material, associated complications which may occur include; hematoma requiring treatment, persistent donor site pain, pelvic instability (iliac crest only), nerve damage with sensory loss, deep or superficial wound infection, herniation (iliac crest only) or excessive bleeding.
- Possible other Adverse Effects associated with use of the TM-S Fusion Device include:
- Abscess,
- Adjacent segment disease,
- Adverse reaction to anesthesia,
- Airway obstruction,
- Allergic reactions to prophylactic antibiotics or blood transfusions,
- Allergic reaction to the implant(s),
- Anesthetic or post-anesthetic reactions,
- Anterior longitudinal ligament penetration,
- Arachnoiditis,
- Arrhythmia,
- Atelectasis,
- Back pain,
- Bladder dysfunction,
- Bone or vertebral fracture during insertion of the device,
- Bone lysis associated with particulate metal debris from the supplemental internal fixation,
- Bone resorption,
- Bursitis,
- Cellulitis,
- Cerebrovascular accident,
- Constipation,
- Cord injury,
- Death,
- Decreased reflexes,
- Deep vein thrombosis,
- Delayed union or nonunion,
- Disc herniation,
- Donor site events (if additional donor site is necessary),
- Dural tear or leak,
- Dysphasia,
- Dysphonia
- Failure of instrumentation,
- Foreign body reaction,
- Graft failure (fracture, resorption, etc.),
- Graft expulsion,
- Great vessel damage,
- Hematoma,
- Hemorrhage,
- Hoarseness,
- Incisional hernia,
- Incisional pain,
- Infection throughout the body (systemic),
- Infection of the wound,
- Ischemia,
- Loss of reduction,
- Malpositioned screws,
- Myelopathy
- Myocardial infarction,
- Neurapraxia,
- Nerve root injury,
- Organ, nerve, blood vessel or muscle damage,
- Osteoporosis local to implant site,
- Pain,
- Painful hardware,
- Paralysis,
- Perforation of the trachea, esophagus or pharynx
- Pneumonia,
- Pseudarthrosis,
- Pulmonary embolism,
- Radiculopathy or lack of improvement in existing radiculopathy,
- Recurrent deformity,
- Reflex sympathetic dystrophy (RSD),
- Scar formation,
- Screw loosening,
- Screw migration,
- Seroma,
- Spinal cord injury,
- Spinal stenosis,
- Spondylolisthesis,
- Subsidence of the implant,
- Swelling,
- Syringomyelia,
- Thromboembolism,
- Thrombophlebitis,
- Thrombosis,
- Tumor formation and/or recurrence,
- Urinary tract infection,
- Vocal chord paralysis,
- Wound dehiscence.
- Additional complications that are not anticipated may also occur.
- Reoperation may be necessary to correct adverse effects.